High-Standard Entry Criteria
TargetMol’s FDA-Approved & Pharmacopoeia Drug Library is built under strict inclusion criteria to ensure that every compound in the collection possesses well-defined structures and exceptional purity, verified through multiple analytical techniques such as NMR, HPLC, and LC-MS.
Through a multi-layer screening mechanism, we have excluded mixtures, polymers, and other compounds with ambiguous structures, as well as inactive substances such as sunscreen agents, contrast agents, and inorganic compounds.
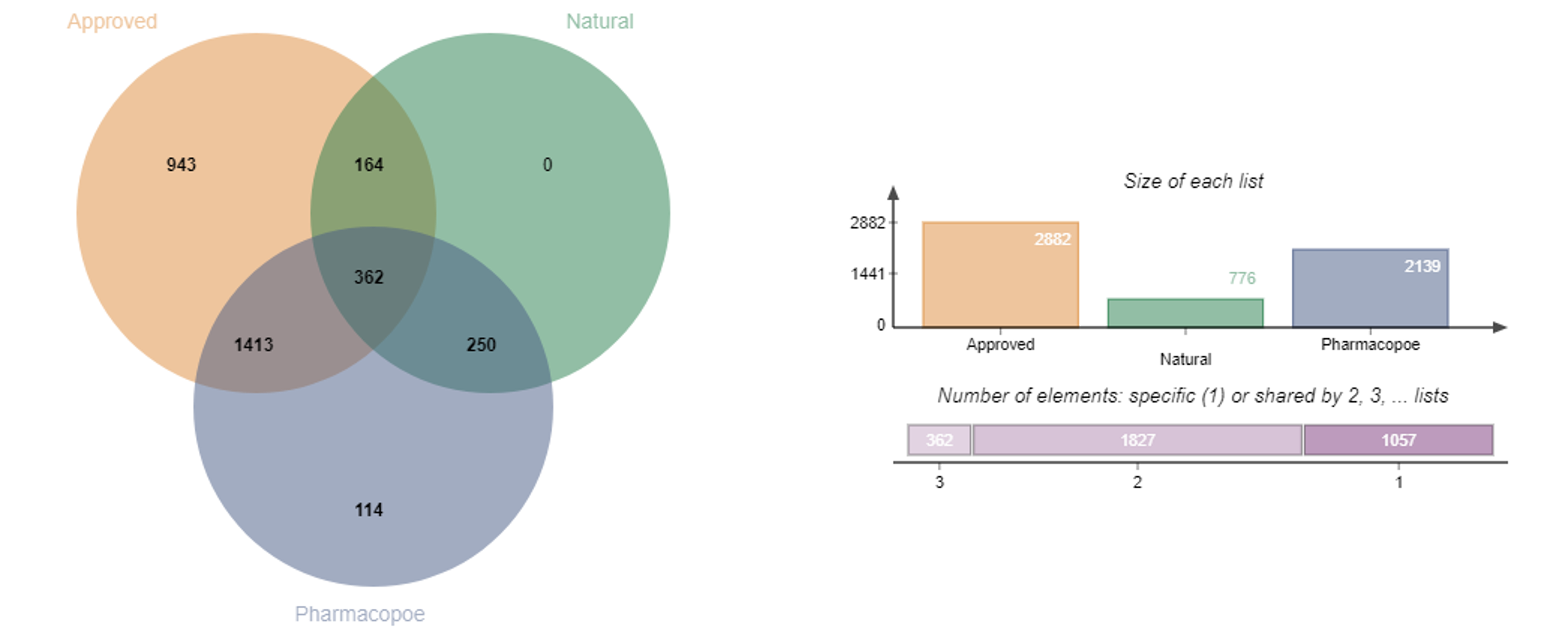
This library includes drugs approved by major regulatory authorities such as the FDA, PMDA, EMA, and NMPA, as well as those listed in various pharmacopoeias.
If you are interested in drugs approved by other organizations, please refer to the following resources:
L9200 Drug Repurposing Compound Library;
L1000 Approved Drug Library;
L1020 EMA Approved Drug Library.
Significant Structural Diversity
The TargetMol FDA-Approved and Pharmacopoeia Drug Library exhibits remarkable structural diversity, offering significant advantages in drug discovery. An analysis of the library based on 85% MACCS fingerprint similarity classifies it into 2,487 categories, covering a broad chemical space. The library contains a wide range of compounds, from simple to complex chemical structures. This diversity provides extensive possibilities for identifying lead compounds with high affinity and specificity for target proteins, greatly facilitating drug innovation. Whether targeting traditional drug targets or emerging, more challenging ones, the FDA-Approved and Pharmacopoeia Drug Library offers abundant candidate compounds, accelerating the drug development process.
Analysis on Compound Diversity
Diverse Compound Selection
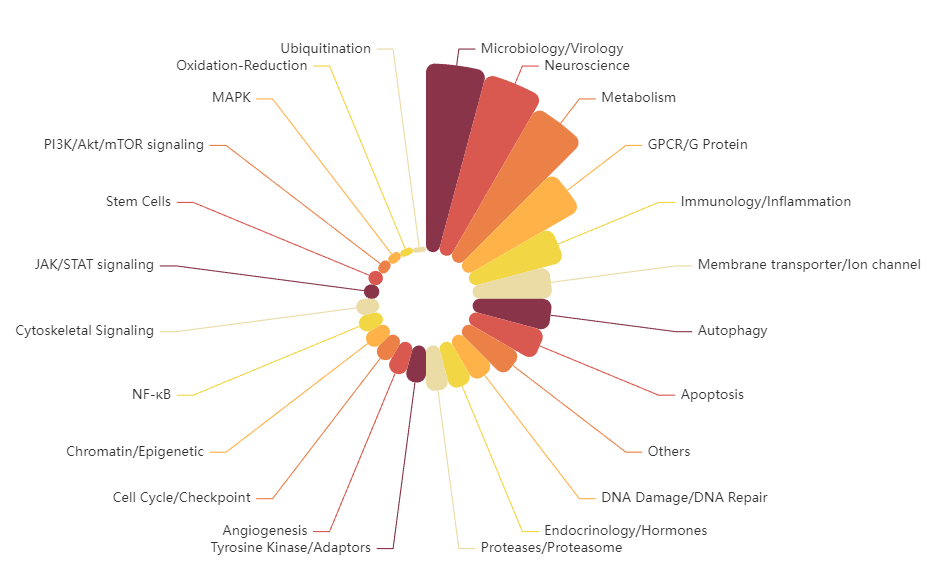
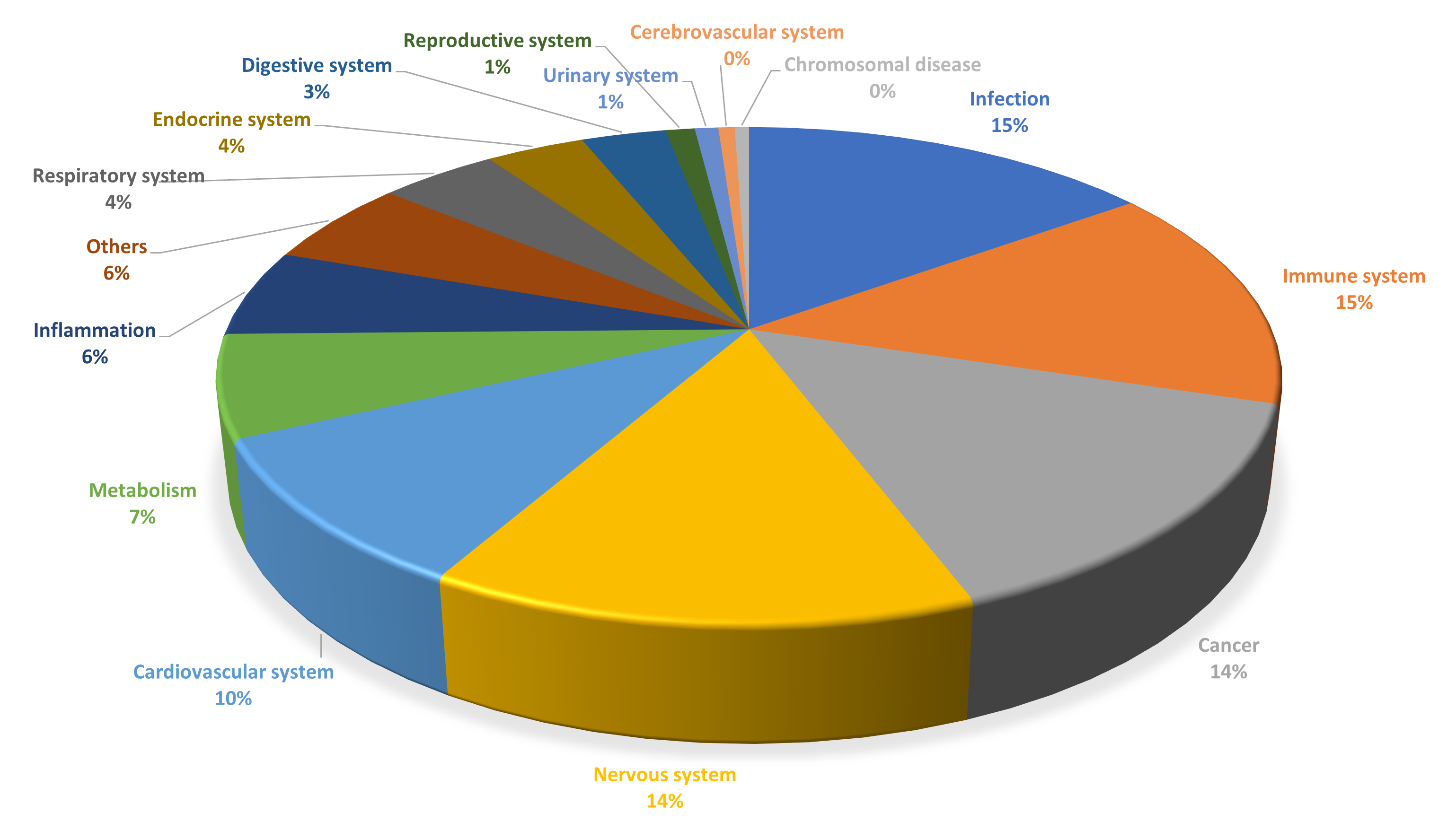
The TargetMol FDA-Approved & Pharmacopeia Drug Library not only exhibits high chemical diversity but also demonstrates broad representation in biological functions. The library contains compounds targeting various key signaling pathways, including kinases, G protein-coupled receptors (GPCRs), ion channels, and epigenetic regulators, comprehensively covering disease areas such as cancer, immune disorders, neurological diseases, metabolic disorders, and infectious diseases. Approximately 27% of the compounds are natural products, including monomeric compounds isolated and identified from plants, animals, and microorganisms.
The FDA-Approved & Pharmacopeia Drug Library is an ideal resource for drug repurposing, lead optimization, and the development of new therapeutic approaches, facilitating the entire process from basic research to translational medicine and accelerating the efficient development of innovative drugs.
Signaling Pathways in Library
Classified by Disease type
Compounds in Library
Regular Updates to Compound Libraries
TargetMol ensures compound libraries stay at the forefront of science by regularly updating our database to include the latest approved and marketed drugs.
Flexible Packaging Options
TargetMol provides a variety of standard packaging sizes (such as 30 μL, 50 μL, 100 μL, 250 μL, and 1 mg), and offer customized packaging solutions tailored to specific needs.
Personalized Custom Services
TargetMol offers fully customized screening services, including the design and synthesis of compound libraries. Our highly flexible service is designed to efficiently meet the unique needs of scientists and researchers.
 Your shopping cart is currently empty
Your shopping cart is currently empty