Shopping Cart
Remove All Your shopping cart is currently empty
Your shopping cart is currently empty
HMGB1 Protein, Mouse, Recombinant (His) is expressed in E. coli.

| Pack Size | Price | USA Warehouse | Global Warehouse | Quantity |
|---|---|---|---|---|
| 5 μg | $129 | - | In Stock | |
| 10 μg | $216 | 20 days | 20 days | |
| 20 μg | $360 | - | In Stock | |
| 50 μg | $543 | - | In Stock | |
| 100 μg | $745 | - | In Stock | |
| 200 μg | $1,070 | 20 days | 20 days | |
| 500 μg | $1,730 | 20 days | 20 days | |
| 1 mg | $2,530 | 20 days | 20 days |
| Biological Activity | Activity has not been tested. It is theoretically active, but we cannot guarantee it. If you require protein activity, we recommend choosing the eukaryotic expression version first. |
| Description | HMGB1 Protein, Mouse, Recombinant (His) is expressed in E. coli. |
| Species | Mouse |
| Expression System | E. coli |
| Tag | N-6xHis |
| Accession Number | P63158 |
| Synonyms | Hmgb1,Hmg-1,Hmg1,High mobility group protein B1,High mobility group protein 1 (HMG-1) |
| Amino Acid | GKGDPKKPRGKMSSYAFFVQTCREEHKKKHPDASVNFSEFSKKCSERWKTMSAKEKGKFEDMAKADKARYEREMKTYIPPKGETKKKFKDPNAPKRPPSAFFLFCSEYRPKIKGEHPGLSIGDVAKKLGEMWNNTAADDKQPYEKKAAKLKEKYEKDIAAYRAKGKPDAAKKGVVKAEKSKKKKEEEDDEEDEEDEEEEEEEEDEDEEEDDDDE |
| Construction | 2-215 aa |
| Protein Purity | > 85% as determined by SDS-PAGE. 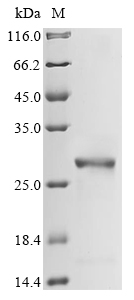 |
| Molecular Weight | 25.7 kDa (predicted) |
| Endotoxin | < 1.0 EU/μg of the protein as determined by the LAL method. |
| Formulation | If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0. |
| Reconstitution | Reconstitute the lyophilized protein in sterile deionized water. The product concentration should not be less than 100 μg/mL. Before opening, centrifuge the tube to collect powder at the bottom. After adding the reconstitution buffer, avoid vortexing or pipetting for mixing. |
| Stability & Storage | Lyophilized powders can be stably stored for over 12 months, while liquid products can be stored for 6-12 months at -80°C. For reconstituted protein solutions, the solution can be stored at -20°C to -80°C for at least 3 months. Please avoid multiple freeze-thaw cycles and store products in aliquots. |
| Shipping | In general, Lyophilized powders are shipping with blue ice. Solutions are shipping with dry ice. |
| Research Background | Multifunctional redox sensitive protein with various roles in different cellular compartments. In the nucleus is one of the major chromatin-associated non-histone proteins and acts as a DNA chaperone involved in replication, transcription, chromatin remodeling, V(D)J recombination, DNA repair and genome stability. Proposed to be an universal biosensor for nucleic acids. Promotes host inflammatory response to sterile and infectious signals and is involved in the coordination and integration of innate and adaptive immune responses. In the cytoplasm functions as sensor and/or chaperone for immunogenic nucleic acids implicating the activation of TLR9-mediated immune responses, and mediates autophagy. Acts as danger associated molecular pattern (DAMP) molecule that amplifies immune responses during tissue injury. Released to the extracellular environment can bind DNA, nucleosomes, IL-1 beta, CXCL12, AGER isoform 2/sRAGE, lipopolysaccharide (LPS) and lipoteichoic acid (LTA), and activates cells through engagement of multiple surface receptors. In the extracellular compartment fully reduced HMGB1 (released by necrosis) acts as a chemokine, disulfide HMGB1 (actively secreted) as a cytokine, and sulfonyl HMGB1 (released from apoptotic cells) promotes immunological tolerance. Has proangiogenic activity. May be involved in platelet activation. Binds to phosphatidylserine and phosphatidylethanolamide. Bound to RAGE mediates signaling for neuronal outgrowth. May play a role in accumulation of expanded polyglutamine (polyQ) proteins.; Nuclear functions are attributed to fully reduced HGMB1. Associates with chromatin and binds DNA with a preference to non-canonical DNA structures such as single-stranded DNA, DNA-containing cruciforms or bent structures, supercoiled DNA and ZDNA. Can bent DNA and enhance DNA flexibility by looping thus providing a mechanism to promote activities on various gene promoters by enhancing transcription factor binding and/or bringing distant regulatory sequences into close proximity. May be involved in nucleotide excision repair (NER), mismatch repair (MMR) and base excision repair (BER) pathways, and double strand break repair such as non-homologous end joining (NHEJ). Involved in V(D)J recombination by acting as a cofactor of the RAG complex: acts by stimulating cleavage and RAG protein binding at the 23 bp spacer of conserved recombination signal sequences (RSS). In vitro can displace histone H1 from highly bent DNA. Can restructure the canonical nucleosome leading to relaxation of structural constraints for transcription factor-binding. Enhances binding of sterol regulatory element-binding proteins (SREBPs) such as SREBF1 to their cognate DNA sequences and increases their transcriptional activities. Facilitates binding of TP53 to DNA. Proposed to be involved in mitochondrial quality control and autophagy in a transcription-dependent fashion implicating HSPB1; however, this function has been questioned. Can modulate the activity of the telomerase complex and may be involved in telomere maintenance.; In the cytoplasm proposed to dissociate the BECN1:BCL2 complex via competitive interaction with BECN1 leading to autophagy activation. Can protect BECN1 and ATG5 from calpain-mediated cleavage and thus proposed to control their proautophagic and proapoptotic functions and to regulate the extent and severity of inflammation-associated cellular injury. In myeloid cells has a protective role against endotoxemia and bacterial infection by promoting autophagy. Involved in endosomal translocation and activation of TLR9 in response to CpG-DNA in macrophages.; In the extracellular compartment (following either active secretion or passive release) involved in regulation of the inflammatory response. Fully reduced HGMB1 (which subsequently gets oxidized after release) in association with CXCL12 mediates the recruitment of inflammatory cells during the initial phase of tissue injury; the CXCL12:HMGB1 complex triggers CXCR4 homodimerization. Induces the migration of monocyte-derived immature dendritic cells and seems to regulate adhesive and migratory functions of neutrophils implicating AGER/RAGE and ITGAM. Can bind to various types of DNA and RNA including microbial unmethylated CpG-DNA to enhance the innate immune response to nucleic acids. Proposed to act in promiscuous DNA/RNA sensing which cooperates with subsequent discriminative sensing by specific pattern recognition receptors. Promotes extracellular DNA-induced AIM2 inflammasome activation implicating AGER/RAGE. Disulfide HMGB1 binds to transmembrane receptors, such as AGER/RAGE, TLR2, TLR4 and probably TREM1, thus activating their signal transduction pathways. Mediates the release of cytokines/chemokines such as TNF, IL-1, IL-6, IL-8, CCL2, CCL3, CCL4 and CXCL10. Promotes secretion of interferon-gamma by macrophage-stimulated natural killer (NK) cells in concert with other cytokines like IL-2 or IL-12. TLR4 is proposed to be the primary receptor promoting macrophage activation and signaling through TLR4 seems to implicate LY96/MD-2. In bacterial LPS- or LTA-mediated inflammatory responses binds to the endotoxins and transfers them to CD14 for signaling to the respective TLR4:LY96 and TLR2 complexes. Contributes to tumor proliferation by association with ACER/RAGE. Can bind to IL1-beta and signals through the IL1R1:IL1RAP receptor complex. Binding to class A CpG activates cytokine production in plasmacytoid dendritic cells implicating TLR9, MYD88 and AGER/RAGE and can activate autoreactive B cells. Via HMGB1-containing chromatin immune complexes may also promote B cell responses to endogenous TLR9 ligands through a B-cell receptor (BCR)-dependent and ACER/RAGE-independent mechanism. Inhibits phagocytosis of apoptotic cells by macrophages; the function is dependent on poly-ADP-ribosylation and involves binding to phosphatidylserine on the cell surface of apoptotic cells. In adaptive immunity may be involved in enhancing immunity through activation of effector T-cells and suppression of regulatory T (TReg) cells. In contrast, without implicating effector or regulatory T-cells, required for tumor infiltration and activation of T-cells expressing the lymphotoxin LTA:LTB heterotrimer thus promoting tumor malignant progression. Also reported to limit proliferation of T-cells. Released HMGB1:nucleosome complexes formed during apoptosis can signal through TLR2 to induce cytokine production. Involved in induction of immunological tolerance by apoptotic cells; its pro-inflammatory activities when released by apoptotic cells are neutralized by reactive oxygen species (ROS)-dependent oxidation specifically on Cys-106. During macrophage activation by activated lymphocyte-derived self apoptotic DNA (ALD-DNA) promotes recruitment of ALD-DNA to endosomes. |
| Size | Quantity | Unit Price | Amount | Operation |
|---|

Copyright © 2015-2026 TargetMol Chemicals Inc. All Rights Reserved.