- Remove All
 Your shopping cart is currently empty
Your shopping cart is currently empty
Shopping Cart
Integrin alpha V beta 8 Protein, Human, Recombinant (His)
Catalog No. TMPY-05717
Integrin alpha V beta 8 is a receptor for fibronectin. It recognizes the sequence R-G-D in its ligands. ITGAVB8 does not appear to assume different activation states; and the cytoplasmic tail does not connect to the cytoskeleton. It binds ligands containing an RGD motif; including vitronectin; fibrin and the latency associated peptide (LAP) of the latent TGF-beta complex. High affinity binding of alpha V beta 8 to LAP allows proteolytic cleavage by MT1-MMP; which releases active TGF-beta. This mechanism differs from that of alpha V beta 6; the other alpha V integrin which can activate TGF-beta from latency through non-proteolytic mechanisms.

Integrin alpha V beta 8 Protein, Human, Recombinant (His)
Catalog No. TMPY-05717
Integrin alpha V beta 8 is a receptor for fibronectin. It recognizes the sequence R-G-D in its ligands. ITGAVB8 does not appear to assume different activation states; and the cytoplasmic tail does not connect to the cytoskeleton. It binds ligands containing an RGD motif; including vitronectin; fibrin and the latency associated peptide (LAP) of the latent TGF-beta complex. High affinity binding of alpha V beta 8 to LAP allows proteolytic cleavage by MT1-MMP; which releases active TGF-beta. This mechanism differs from that of alpha V beta 6; the other alpha V integrin which can activate TGF-beta from latency through non-proteolytic mechanisms.
| Pack Size | Price | Availability | Quantity |
|---|---|---|---|
| 100 μg | $517 | In Stock | |
| 200 μg | $913 | 7-10 days | |
| 500 μg | $1,930 | 7-10 days |
Bulk & Custom
Add to Cart
All TargetMol products are for research purposes only and cannot be used for human consumption. We do not provide products or services to individuals. Please comply with the intended use and do not use TargetMol products for any other purpose.
Select Batch
Resource Download
Product Information
| Biological Activity | Activity testing is in progress. It is theoretically active, but we cannot guarantee it. If you require protein activity, we recommend choosing the eukaryotic expression version first. |
| Description | Integrin alpha V beta 8 is a receptor for fibronectin. It recognizes the sequence R-G-D in its ligands. ITGAVB8 does not appear to assume different activation states; and the cytoplasmic tail does not connect to the cytoskeleton. It binds ligands containing an RGD motif; including vitronectin; fibrin and the latency associated peptide (LAP) of the latent TGF-beta complex. High affinity binding of alpha V beta 8 to LAP allows proteolytic cleavage by MT1-MMP; which releases active TGF-beta. This mechanism differs from that of alpha V beta 6; the other alpha V integrin which can activate TGF-beta from latency through non-proteolytic mechanisms. |
| Species | Human |
| Expression System | HEK293 Cells |
| Tag | C-His |
| Accession Number | P06756&P26012 |
| Synonyms | Integrin α V β 8,Integrin alpha V β8 |
| Construction | A DNA sequence encoding the extracellular domain (Met1-Val992) of human ITGAV (P06756) was fused with a C-terminal polyhistidine tag was expressed., constructed the plasmid 1; A DNA sequence encoding the extracellular domain (Met1-Arg684) of human ITGB8 (P26012) constructed the plasmid 2. The two plasmids were co-expressed and the human ITGAV&ITGB8 heterodimer was purified. Predicted N terminal: Phe31 & Glu43 |
| Protein Purity | ≥ 95 % as determined by SDS-PAGE. ≥ 95 % as determined by SEC-HPLC. 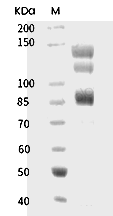 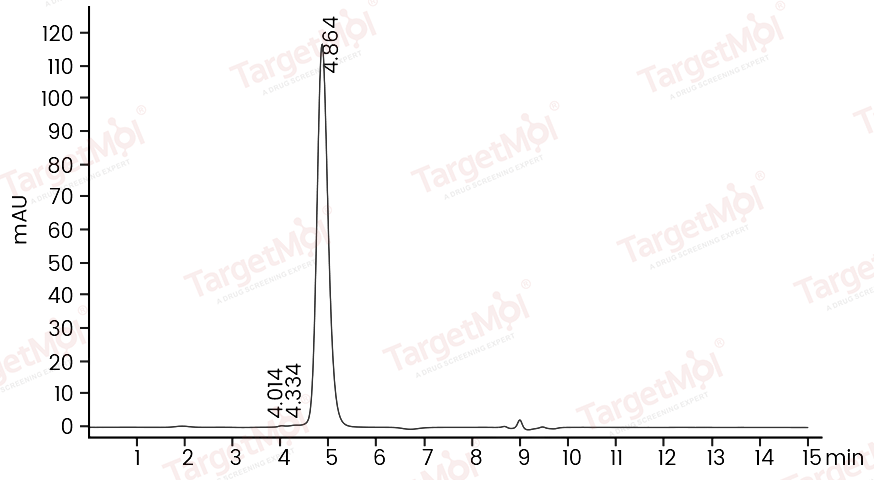 |
| Molecular Weight | 187.95 kDa (predicted) |
| Endotoxin | < 1.0 EU/μg of the protein as determined by the LAL method. |
| Formulation | Lyophilized from a solution filtered through a 0.22 μm filter, containing PBS, pH 7.4. Typically, a mixture containing 5% to 8% trehalose, mannitol, and 0.01% Tween 80 is incorporated as a protective agent before lyophilization. |
| Reconstitution | A Certificate of Analysis (CoA) containing reconstitution instructions is included with the products. Please refer to the CoA for detailed information. |
| Stability & Storage | It is recommended to store recombinant proteins at -20°C to -80°C for future use. Lyophilized powders can be stably stored for over 12 months, while liquid products can be stored for 6-12 months at -80°C. For reconstituted protein solutions, the solution can be stored at -20°C to -80°C for at least 3 months. Please avoid multiple freeze-thaw cycles and store products in aliquots. |
| Shipping | In general, Lyophilized powders are shipping with blue ice. |
| Research Background | Integrin alpha V beta 8 is a receptor for fibronectin. It recognizes the sequence R-G-D in its ligands. ITGAVB8 does not appear to assume different activation states; and the cytoplasmic tail does not connect to the cytoskeleton. It binds ligands containing an RGD motif; including vitronectin; fibrin and the latency associated peptide (LAP) of the latent TGF-beta complex. High affinity binding of alpha V beta 8 to LAP allows proteolytic cleavage by MT1-MMP; which releases active TGF-beta. This mechanism differs from that of alpha V beta 6; the other alpha V integrin which can activate TGF-beta from latency through non-proteolytic mechanisms. |
Dose Conversion
You can also refer to dose conversion for different animals. More
Sci Citations
Calculator
Tech Support
Please read the User Guide of Recombinant Proteins for more specific information.

Copyright © 2015-2025 TargetMol Chemicals Inc. All Rights Reserved.


